58+ calculate the molality of the following aqueous solutions
The volume of a solution on the other hand is slightly dependent upon temperature. Molality 05 moles 10 kg 05 molal.
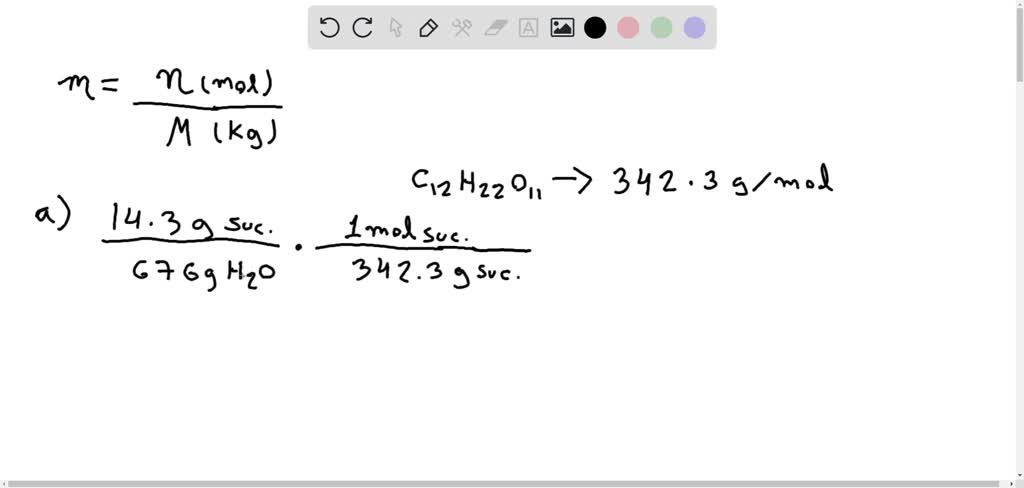
Solved Calculate The Molality Of Each Of The Following Solutions A 14 3 G Of Sucrose C12 H22 O11 In 676 G Of Water B 7 20 Moles Of Ethylene Glycol C2 H6 O2 In
5844 g 5844 grmol 100 mol.

. Molarity 015 moles of KMnO 4 075 L of solution. Bartleby Chemistry Physical Properties Of Solutions. Round your answer to 2 signiflcant digits.
In order to be a true solution a mixture must be stable. 5826 mol 07576 kg 7690 m. A 122 M sugar C 12 H 22 O 11 solution density of solution 112 gmL b 087 M NaOH solution density of solution 104 gmL c 524 M NaHCO 3 solution density of solution 119 gmL.
Calculate the molality of each of the following aqueous solutions. Text Molarity dfrac text mol solute text L of solution Molarity L of solutionmol solute. The solution to this problem involves two steps.
1329 gcm3 times 1000 cm3 1329 g the mass of the entire solution 1329 g minus 5714 g 7576 g 07576 kg the mass of water in the solution 5714 g 980768 gmol 5826 mol of H2SO4. Calculate the molalities of the following aqueous solutions. A 368 M NaCl density of solution 108 gmL b 572 percent by mass KBr solution.
C 524 M NaHCO3 solution density of solution 119 gmL. A Concentration 122 M density 112 gml Process 1- Calculate the moles of sugar Molarity moles 1L then 122 moles 1L Number of moles 122 2- Calculate the mass of solution density massvolume mass density x volume mass 112kgL x 1 l mass 112 kg 3-. Web Molality moles of solute kg of solvent.
Using molality formula we have. You can also verify the results by. Calculate the molalities of the following aqueous solutions.
Molarity 020 M. Web Calculate the molality of each of the following solutions. Web Bookmark File Calculate The Molality Of Each Following Aqueous Solutions Read Pdf Free Report of Investigations Chemistry.
21 Jan 25 2014 How can you convert between molarity and molality. The solution density is required. Determine the molality of a solution prepared by dissolving 2860 g of glucose C 6 H 12 O 6 into 250 g of water.
The molarity of this solution is 020 M moles per liter. 1 Litre of Solution 1000cm3 1000mL. The Central Science Journal of the American Chemical Society Transactions of the Faraday Society Problems in Physical Chemistry JEE Main and Advanced Volume 2 Chemistry Class XII For Madhya Pradesh Board by Dr.
0710 kg of sodium carbonate washing soda Na 2 CO 3 in 100 kg of watera saturated solution at 0C 125 g of NH 4 NO 3 in 275 g of watera mixture used to make an instant ice pack 25 g of Cl 2 in 125 g of dichloromethane CH 2 Cl 2. Kg water 100 g solution - 42 g KBr 958 g H2O 00958 kg H2O. A 583 g of H2SO4 in 150 kg of waterthe acid solution used in an automobile battery b 086 g of NaCI in 100102 g of watera solution of sodium chloride for intravenous injection c 4685 g of codeine C18H21NO3 in 1255 g of ethanol C2H5OH d 25 g of I2 in 125 g of.
Web Since the molar mass gram formula mass of sodium chloride is 58 grams per mole Na 23 g and Cl 35 g 23 35 58 gmol the mole value of the NaCl is 05 moles 29 g 58 gmol 05 moles. Web Molality m moles of solute per kg of solvent not to be confused with molarity 1a 42 mm 42 g KBr100 g solution. Molality moles of solute kg of solvent.
Divide moles by kg of solvent to get molality. Molarity moles soluteLiter solution. Web Liters of solution mL of solution x 1 L1000 mL Liters of solution 750 mL x 1 L1000 mL Liters of solution 075 L.
This is enough to calculate the molarity. Web The answer to your question is below Explanation. In the above problem 5844 gramsmol is the molar mass of NaCl.
Part 2 of 2 426 percent by mass KBr solution. Calculate the molality of each of the following aqueous solutions. A 250 M NaCl solution density of solution 108 gmL b 482 percent by mass KBr solution.
The percent by mass of methanol MM 3204 gmol in an aqueous solution is 223. A solution of 085 g of an organic compound in 1000 g of benzene has a freezing point of 516. The dissolved substances in an aqueous solution may be solids gases or other liquids.
Round your answer to 3 significant digits. Convert grams to moles. Web Molality is used because its value does not change with changes in temperature.
Web Transcribed image text. Web Calculate the molality of each of the following aqueous solutions. An aqueous solution is water that contains one or more dissolved substance.
Moles KBr 42 g KBr x 1 mol KBr119 g 00353 moles KBr. Web Calculate the molality of each of the following solutions. A 122M sugar C12H22O11 solution density of solution 112 gmL.
We need moles KBr and kg of water so we proceed as follows. Web What would be the molality of the solution. A 368 M NaCl density of solution 108 gmL b 572 percent by mass KBr solution.
Formula Tf iKfm 516 1 x 512 x m m 1008 molkg Here m is molality of solution. Part 1 of 2 282MNaCl density of solution 108mLg. Click to see the answer.
Web In this chapter we will focus on solution where the solvent is water. Molality 05 moles 10 kg 05 molal calculating molality of a solution BRIAN M. The mass of water is 1000 grams which is converted to 10 kg.
Web Molarity or molar concentration is the number of moles of solute per liter of solution which can be calculated using the following equation. Web Calculate the molality of each of the following aqueous solutions. B 087 M NaHO solution density of solution 104 gmL.
Expert Solution Want to see the full answer. Web Calculate the molalities of the following aqueous solutions.

Oneclass Calculate The Molality And Van T Hoff Factor I For The Following Aqueous Solution 0 700

Mole Fraction Of Urea In Its Aqueous Solution Is 0 2 Them Molality Of The Solution Will Be
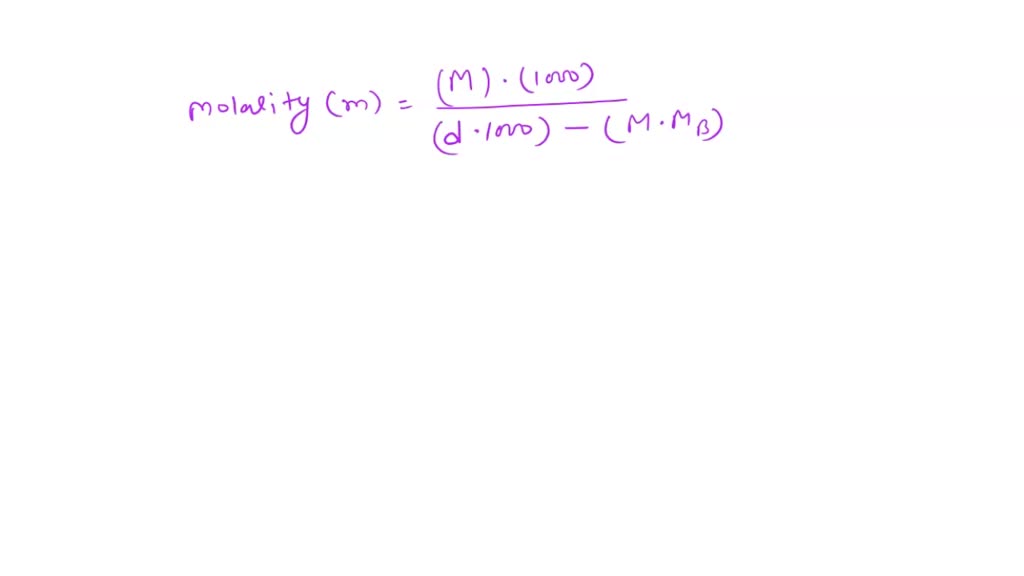
Solved Be Sure To Answer All Parts Calculate The Molalities Of The Following Aqueous Solutions A 1 09 M Sugar C12h22o11 Solution Density Of Solution 1 12 G Ml B 0 703 M Naoh Solution

Which Of The Following Aqueous Solution Has Highest Freezing Point
Answer In General Chemistry For Kelly 305052

Chapter 2 Solutions
Answer In General Chemistry For Kelly 305053
Calculate Molality Of An Aqueous Solution Where Moles Of Solute Are 0 4

Molality Formula Examples How To Calculate Molality Video Lesson Transcript Study Com

6 Calculate The Molalities Of The Following Aqueous Solutions A 0 840 M Sugar C12h22o11 Solution Brainly In
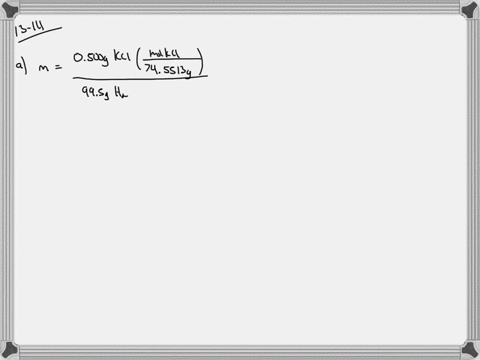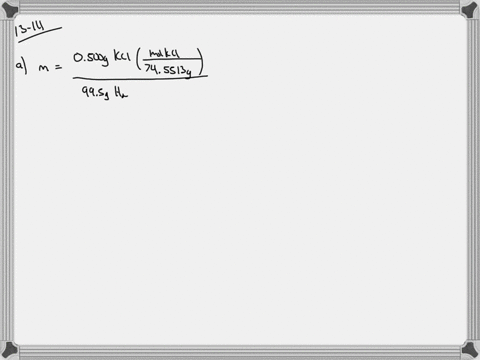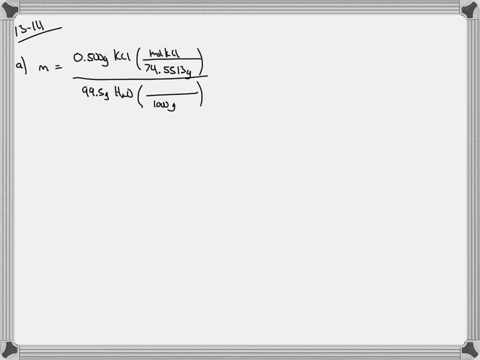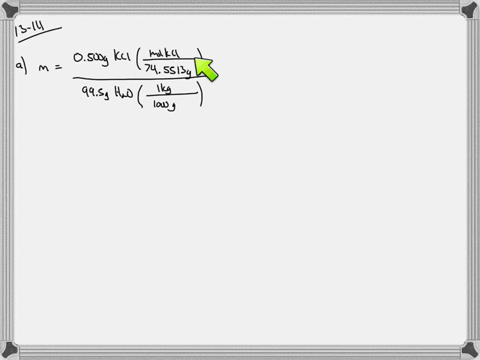
Solved Calculate The Molality And Van T Hoff Factor I For The Following Aqueous Solutions A 0 500 Mass Kcl Freezing Point 0 234 C B 1 00 Mass H2 So4 Freezing Point 0 423 C

Oneclass 4 Points Calculate The Molarity Of The Following Aqueous Solution 22 4 G Of Licio 3 H 0

Calculated The Molality Of A Sulphuric Acid Solution In Which The Mole Fraction Of Water Is 0 85 Youtube
The Mole Fraction Of Solute In Aqueous Solution Is 0 2 Calculate Molality Of Solution
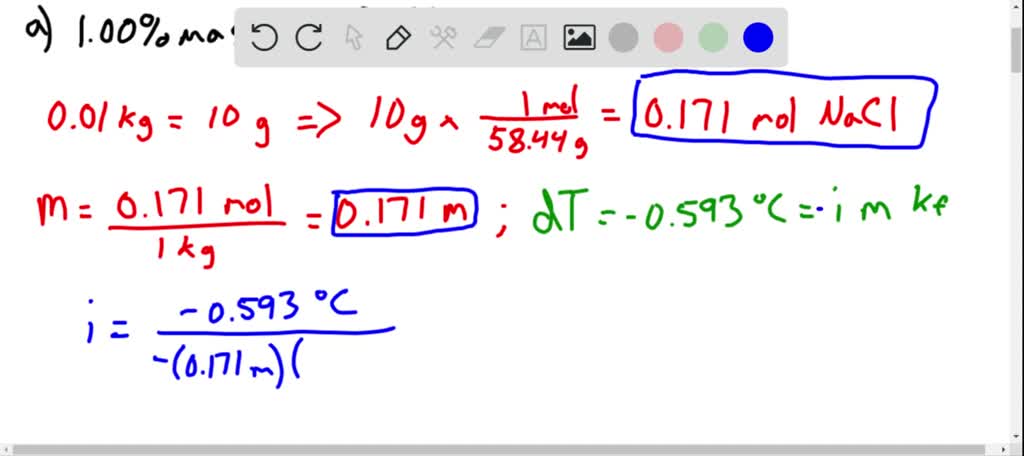
Solved Calculate The Molality And Van T Hoff Factor I For The Following Aqueous Solutions A 1 00 Mass Nacl Freezing Point 0 593 C B 0 500 Mass Ch3 Cooh Freezing Point 0 159 C

Calculate The Mole Fraction And Molality Of Hno3 In A Solution Containing 12 2

Which Of The Following 0 1 M Aqueous Solutions Will Have The Lowest Freezing Point Youtube